The advent of disruptive biologic technologies (e.g. Kymriah, Zolgensma- Novartis; Humira; AbbVie; Keytruda- Merck; Reolysin- Oncolytics Biotech, etc.) has ushered in an unprecedented need for the highly specialized field of biotherapeutics manufacturing. In a pre-COVID-19 world, there existed a critical, unmet global demand for access to biotherapeutics manufacturing capacity and expertise which has been compounded exponentially with the emergence of the pandemic. Since the pandemic onset, Canada has targeted upwards of $170 million in investments to increase the domestic manufacturing capacity required to support a national vaccine production initiative in the coming months. Prior to COVID-19, competition for highly skilled staff in the area of biomanufacturing was high; in the era of COVID-19, this need is reaching a breaking point. Although the current COVID-19 requirement for trained personnel is immediate, to ensure the sustainability of the investments that Canada is making in domestic manufacturing beyond the pandemic, it is important to build a tangible pipeline of job-ready, highly qualified personnel (HQP) to support this newly established, expanded capacity.
From its inception in 2014, BioCanRx has committed to providing the HQP within its network with access to unique and sought-after training opportunities to supplement their existing expertise. BioCanRx is a federally funded, Network of Centres of Excellence with a mandate of accelerating novel cancer immunotherapies into the clinic for the benefit of Canadian cancer patients. In Cycle 1 of its funding, BioCanRx was very successful in delivering on its mandate. This is owing in large part to the purposeful, iterative approach BioCanRx takes to integrate technology development, biomanufacturing and clinical translation. This includes representation from patients and patient stakeholders, and is supported by targeted training initiatives for highly qualified personnel, which to date, has led to the initiation of 12 clinical trials and the inception of 24 new, made-in-Canada biotechnologies. Recognizing early on the critically important role that biomanufacturing and, by extension, the biomanufacturing workforce played in the successful translation of novel immunotherapies, BioCanRx recently announced a new strategic priority for Cycle 2 funding to scale out and expand biomanufacturing capacity in Canada. In support of this strategic priority, BioCanRx has committed more than 30% of its Cycle 2 funding envelope to bolster current domestic vector manufacturing capacity, and scale out a first-of-its-kind, point-of-care (POC) manufacturing network to enable the translation of novel cell and gene therapies such as CAR T-cells used in the BioCanRx-funded CLIC1901 clinical trial to treat refractory leukemia and lymphoma in patients across Canada.
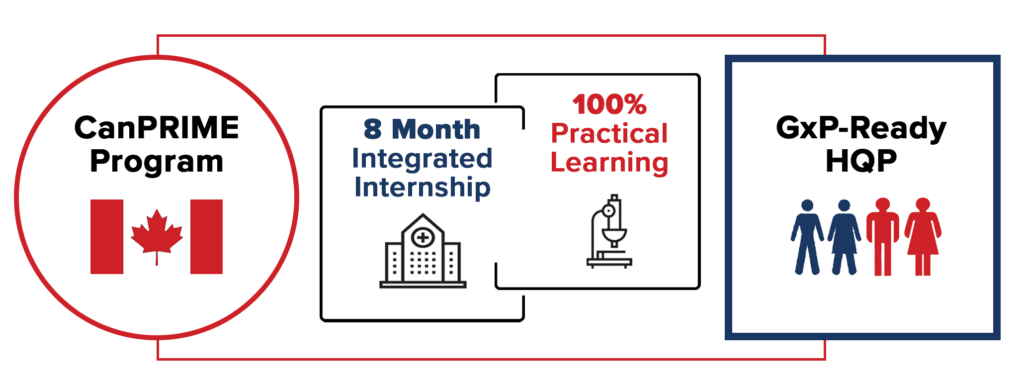
To support the critical need for job-ready HQP in the biomanufacturing sector, BioCanRx is leveraging its involvement with an established, first in Canada, hands-on training program currently ongoing at The Ottawa Hospital’s Biotherapeutics Manufacturing Centre (BMC), in support of BioCanRx’s POC network initiative. Known as the Canadian Partnership for Research in Immunotherapy Manufacturing Excellence or CanPRIME, this unique training program brings together the complementary offerings of college (Algonquin College) and university (University of Ottawa) training, with invaluable industry expertise, guidance and funding partnered with support from the Mitacs Accelerate Cluster Program. Key to the success of CanPRIME has been the BMC, a GMP facility housed at The Ottawa Hospital, that has an unrivalled track record of success in GMP manufacturing therapeutic viruses for clinical use in Asia, Canada, Europe and the USA. Importantly, the BMC is a crossroad between industry and academia making it an ideal location to bring together the contributions of the partners listed here and to transform them into a truly unique and comprehensive training environment spanning the entirety of the biomanufacturing continuum. Established in May 2019, and for the following 5 years, CanPRIME will support the onboarding and training of more than 30 trainees from university and college at the BMC, creating a pipeline of HQP armed with invaluable experience and marketability for future employment opportunities within the biomanufacturing sector. Employability of its ‘graduate’ interns currently sits at 100%, demonstrating both the need and high employability of these GMP-ready individuals.
The knowledge that is required to increase biomanufacturing capacity and expertise in Canada is the functional understanding and proper implementation of GxP; Good Manufacturing Practices (GMP), for example. GxP is a collection of quality guidelines and regulations created to ensure that biopharmaceutical products are safe, meet their intended use and adhere to quality specifications during manufacture, control, storage and distribution. While traditional, academic training (university and college) may touch on aspects of GxP, CanPRIME provides the essential hands-on experience and know-how required for job-readiness and on-the-ground implementation of GxP. Following in the footsteps of the inaugural CanPRIME program, the BioCanRx-led new CanPRIME 2.0 will likewise marry the complementary expertise gained in University and College classrooms to GMP sites across Canada to support the initiation and sustainability of its POC network through an integrated learning approach.
As CanPRIME aims directly at imparting comprehensive professional competence in GMP biomanufacturing, it serves to greatly reduce onboarding times of these highly sought-after individuals, a major value-add for prospective employers. CanPRIME has and will continue to be guided by the requirements of the labour market and the needs of its interns to acquire the real-life skills and competencies to prove themselves as assets in the Canadian biomanufacturing ecosystem. During this challenging period in our history, ensuring this critical on-the-job skillset is gained by HQP has never been more important to securing our short, medium and long term biomanufacturing domestic capacity and scale up of our ecosystem.
It is clear that there is a real and pressing global need for capacity to manufacture biologics for clinical use. BioCanRx recognizes and understands the critical importance that unique training offerings like CanPRIME are to anchoring biomanufacturing in Canada. While a number of complimentary training programs directed at GxP exist, CanPRIME and its next iteration, CanPRIME 2.0, represent a unique and novel approach to provide experienced, job-ready individuals to provision industry, academia and not-for-profits with GMP advanced therapeutic product biomanufacturing know-how.